Oncologica‘s genetic tests can identify patients who are most likely to respond to Immunotherapies and tap into the full potential of genome-based medicine.
What is
Immunotherapy?
Immunotherapy is an anti cancer treatment that stimulates the reaction of our own immune system to cancer cells and is now a front line treatment for many tumour types.
-1500w.png)
Immunotherapy drugs have been proven to be effective in prolonging the lives of patients with advanced cancers while also having far less toxic side effects than chemotherapy.
Does Immunotherapy work for everyone?
Immunotherapy doesn’t work for everyone: in some patients it appears to work incredibly well, completely destroying tumours even after they have begun to spread around the body, but for other patients the response can be less dramatic.Therefore, in routine clinical practice it is necessary to establish if the patient will respond to immunotherapy treatments or not.
Oncologica’s Immunofocus genetic tests can identify patients who are most likely to be able to tap into this type of genome-based cancer medicine.
-min-1400w.png)
Oncologica®’s Immunofocus Solutions
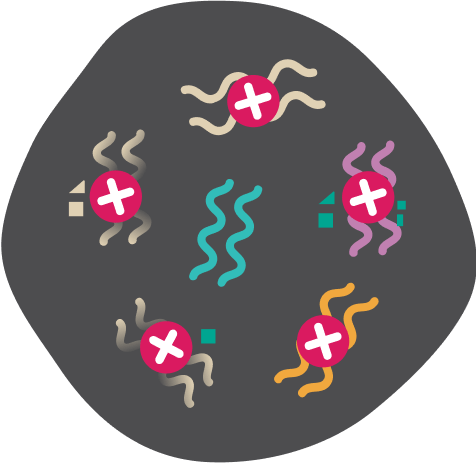
Immunofocus TMB Test assesses the number of mutations (and the related number of neoantigens) in the cancer cell. The higher the tumour mutational burden, the higher the chance for the individual to respond to Immunotherapy.

1.
Multiple DNA mutations occur in the tumour cell
2.
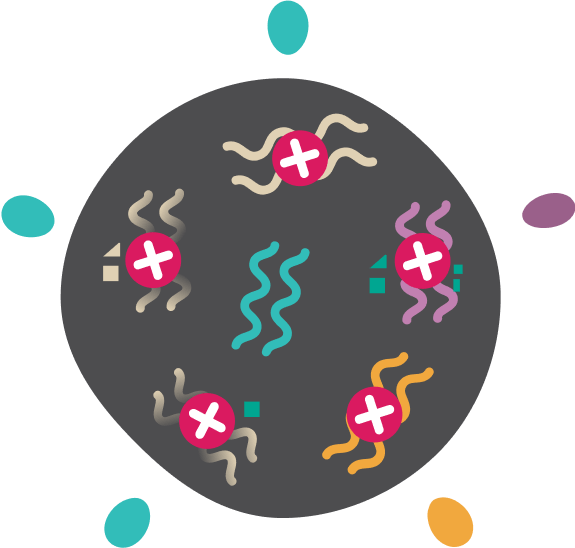
Mutations create neoantigens which are recognised for targeted killing by immune system
3.
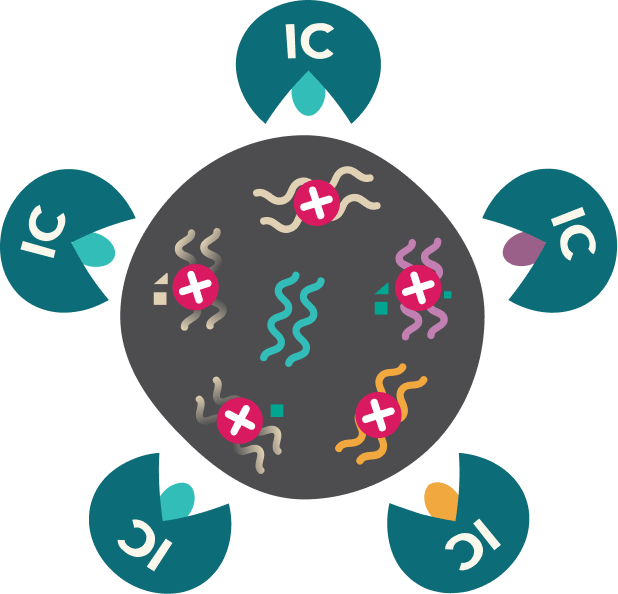
Immunotherapy activates the immune cells to target the neoantigens and kill the tumour cell
-1000w.png) Tumour mutational burden (TMB) is a biomarker test for multiple disease types that together with Oncofocus DNA tests provide truly comprehensive tumour sample immunotherapy informatics. Faster genetic disease information can speed you to your next steps.
Tumour mutational burden (TMB) is a biomarker test for multiple disease types that together with Oncofocus DNA tests provide truly comprehensive tumour sample immunotherapy informatics. Faster genetic disease information can speed you to your next steps.The Immunofocus TMB test is performed on routine biopsy samples to assess the number of DNA mutations in a tumour cell’s genome, which can be used to predict the response to immunotherapy.
Immunofocus PD-L1 Test measures the expression levels of PD-L1 protein.
A range of cancer types have been shown to bind PD-L1 with the PD-1 of immune cells (t-cells).
This blocks the activation of the t-cell, allowing the tumour cell to evade destruction. The cancer types effected include:
A range of cancer types have been shown to bind PD-L1 with the PD-1 of immune cells (t-cells).
This blocks the activation of the t-cell, allowing the tumour cell to evade destruction. The cancer types effected include:
- Melanoma
- lung
- head and neck
- stomach
- ovarian
-min-1200w.png)
Targeting the PD-1/PD-L1 connections with inhibitors has emerged as a powerful immunotherapy against these cancer types. Inhibiting the PD-1/PD-L1 pathway with therapeutic antibodies results in restoration of immune responses and activation of
t-cells directed to kill the tumour cell.
This therapy has been shown to induce a strong clinical response in many tumour types, for example 20-40% in melanoma and 33-50% in advanced non small cell lung cancer (NSCLC).

Malfunctioning of the Mismatch Repair (MMR) pathway increases the mutational burden of specific cancers and is often involved in the cause of the malfunction, sometimes as an influential bystander and sometimes as the main driving force.
Immunofocus MMR Test analyses the Mismatch Repair genes (MLH1, MSH2, MSH6 and PMS2) to identify DNA repair defects linked to response to immunotherapy. The MMR test quickly identifies whether or not a new class of medicines will benefit you.
The MMR genetic test results provide the data needed to get access to the medicines through your oncologist or a clinical trial as quickly as possible. If the tests show that immunotherapy will benefit you, then the sooner you begin treatment the sooner you can start feeling better.
Immunofocus MMR Test analyses the Mismatch Repair genes (MLH1, MSH2, MSH6 and PMS2) to identify DNA repair defects linked to response to immunotherapy. The MMR test quickly identifies whether or not a new class of medicines will benefit you.
The MMR genetic test results provide the data needed to get access to the medicines through your oncologist or a clinical trial as quickly as possible. If the tests show that immunotherapy will benefit you, then the sooner you begin treatment the sooner you can start feeling better.
-600w.png)
Why Choose Immunofocus?
By determining the patients responsiveness to our Immunotherapy solutions, Oncologica’s Immunofocus DNA tests can help patients find the therapy option that will work best for their tumour.Immunofocus also helps patients avoid medication that will have little effect on their tumour and the side effects that could come from these types of therapy.
-min-200h.png)